Accelerate Your Clinical Research from Data Collection to Breakthrough
StudyLINQ streamlines every aspect of clinical trial management so research teams can focus on what matters most: advancing medical science.


Clinical Trial Management Shouldn’t Slow Down Your Research
Research teams face mounting challenges:
Juggling multiple spreadsheets and disconnected systems
Hours spent on manual data entry adn case report generation
Risk of compliance issues with inadequate adverse event tracking
Difficulty coordinating multi-site trials across institutions
Lost time following up with potential participants
Every hour spent on administrative tasks is an hour not spent advancing your research.
A Complete Clinical Trial Management System
Built for Research Teams
StudyLINQ is a comprehensive CTMS that centralizes every aspect of trial management, from participant recruitment through data analysis. Built on ITW's proven data collection platform, StudyLINQ eliminates administrative bottlenecks so you can focus on your research objectives.

What Makes StudyLINQ Different
Effortless Participant Tracking
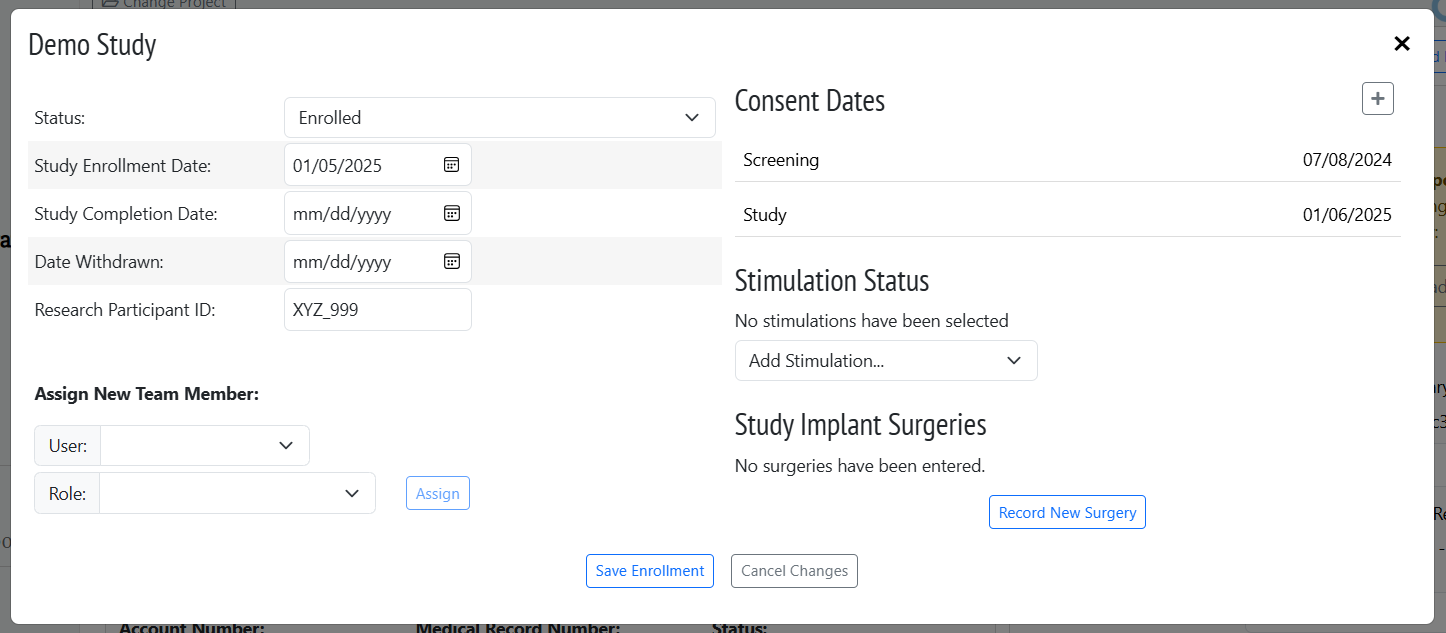
See exactly where each participant stands in your study at a glance. Our intuitive Study Dashboard gives you complete visibility into enrollment status, assessment completion, and study progress — no more hunting through spreadsheets!

Automated Reporting That Saves Hours
Generate case report forms automatically using customizable templates that pull participant data directly from assessments. What used to take hours now takes minutes.
Built-In Safety & Compliance
Comprehensive adverse event tracking ensures potential issues are immediately flagged and routed to the right team members. Stay compliant with confidence while protecting participant safety.

Streamlined Independent Reviews
Enable blinded data analysis for independent reviewers from collaborating facilities. Reviewers access de-identified data securely within the system, and their results automatically link back to original assessments—no data exports or security risks.
Intelligent Randomization
Let StudyLINQ handle study group assignments using the Pocock-Simon covariate adaptive randomization method. The system automatically balances distributions based on your predetermined strata — no manual calculations required.
Organized Recruitment Pipeline
Our Inquiry system transforms recruitment from chaos to clarity. Collect detailed candidate information, use customizable worklists to identify qualified prospects, and set up automatic follow-up reminders. Perfect for multi-site trials.

Built for Teams Leading Breakthroughs in Healthcare
Research Facilities & Organizations
Medical Research Facilities
Hospitals & Clinics
Research Centers
Universities
Research Team Members
Principal Investigators
Study Coordinators & Leads
Regulatory Coordinators
Research Physicians
Research Nurses
Ready to Streamline Your Clinical Trials?
No obligation. See the platform in action with your use case.
See how StudyLINQ can transform your research workflow.